The Periodic Table
Short Questions
Question 1: State Newland’s law of octaves. Why did the law of Octaves fail ? Write three important drawbacks of Newland’s classification.
Answer: Newland’s Law of Octaves: The elements with similar properties occur each time after every seven elements. Just the same way as the repetition of musical node in a octave.
The law failed because of the following reasons:
(1) The law was applicable only upto calcium. It could not include the other elements beyond calcium.
(ii) With the discovery of rare gases, it was the ninth element and not the eighth element having similar chemical properties.
Drawbacks:
(i) Newland found that elements could not be fitted into the table.
(ii) Atomic weights were not correctly estimated.
(iii) Certain elements did not fit into the scheme.
Question 2: Answer the following questions regarding Mendeleev’s modified Periodic Table.
(î) How many periods are there in Mendeleev’s Periodic Table?
(iii) How many groups consist of normal elements and transitional elements in Mendeleev’s Periodic Table?
(iv) Which group of elements was missing from Mendeleev’s original Periodic Table?
(v) What is the number of first and last vertical column?
(vi) in which vertical column hydrogen is placed.
(vii) What are horizontal rows known as ? How many are they in number?
(viii) How many elements are there in the first horizontal row?
(ix) How many elements are there in the second and third horizontal rows each?
Answer: (i) There are seven periods in Periodic Table.
(ii) There are nine groups, numbered from 0 to 8.
(iii) The groups IA, IIA, IIIA. IVA, VA, VIA and VILA consist normal elements and groups, IIIB, IVB, VB, VIB, VIIB and IB, IIB consist transition elements.
(iv) Zero group (Noble gases).
(v) The first column is numbered zero, whereas last group is numbered 8.
(vi) Hydrogen is placed in IA group.
(vii) The horizontal rows in Periodic Table are known as periods. There are seven periods in all.
(viii) There are only two elements in the first period.
(ix) The second and third periods have eight elements each.
Question 3: What is modem periodic law ? Name the elements of ‘first transition series’.
Answer: The modern periodic law states that—the properties of elements are the periodic functions of their electronic configuration”. Scandium, Titanium, Vanadium, Chromium, Manganese, Iron, Cobalt, Nickel, Copper, Zinc.
Question 4: Who gave modern periodic law? What is meant by a Group in the Periodic Table?
Answer: Henry Moseley. Vertical column in a Periodic Table are called groups.
Question 5: How many vertical columns are there in the modern periodic table ? State whether the ionization potential increases or decreases on going down a Group.
Answer: 18. Ionization potential decreases on going down a group.
Question 6: How many elements are in the second period of modem periodic table ? Write the names of first and last elements of second period.
Answer: There are eight elements in the second period of the periodic table. Lithium (Li) and Neon (Ne) are the first and last element of the second period.
Question 7: How many elements are in the third period of modern periodic table ? Write the names of first and last elements of third period.
Answer: Eight elements in the third period of the periodic table. Sodium (Na) and Argon (Ar) are the first and last elements of the third period.
Question 8: How many elements are in the fourth, fifth and sixth period of modem periodic table?
Answer: There are 18, 18 and 32 elements in the fourth, fifth and sixth period of the periodic table respectively.
Question 9: Which period is the shortest one ? In which period maximum number of elements are present?
Answer: The first period is the shortest period in the periodic table as it have only two elements i.e., H and He. The period in which the maximum number of elements are present in 6th period. It is the longest period of the periodic table as it have 32 elements.
Question 10: Give the number of the group and the period, of the clement having three shells with three electrons in valence shell.
Answer: The element having three shells with three electrons in valence shell belong to 13th group and 3rd period.
Question 11: What are the main characteristic of the last elements in the Periodic Table? What is the general name given to such elements?
Answer: The main characteristic of the last elements in the periods is the presence of 8 electrons in their valence shell (i.e., octet is complete). The general name of such elements are noble gases or inert gases or rare gases or aerogens.
Question 12: What are typical elements ? Give the liquid elements in Periodic Table.
Answer: Na, Mg, Al, Si, P, S, Cl, Ar all elements present in third short period in Periodic Table. These elements are known as typical elements. Francium, Mercury and Bromine are known as liquid elements.
Question 13: What are iso-electronic ions ? Account for the decrease in size of the following iso-electronic ions:
O2- > F– > Mg2+ > Al3+
Answer: Iso-electronic ions are of different elements having the same number of electrons but differ from one another in magnitude of nuclear charge. The order of decrease in size is as follows :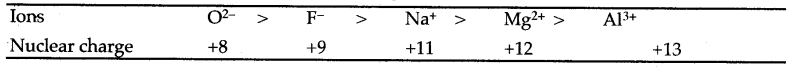
On moving from left to right, the nuclear charge goes on increasing and the electrons are pulled more and more strongly decreasing the size of the ions.
Question 14: Give the formula of one species positively charged and one negatively charged that will be iso-electronic with Ne.
Answer: Cation iso-electronic to neon = Mg2+.
Anion iso-electronic to neon = F–.
Question 15: (1) State the number of elements in Period 1, Period 2, and Period 3 of the Periodic Table.
(ii) Name the elements in Period 1.
(iii) What happens to atomic size of elements on moving from left to right in a period?
Answer: (i) Period contains 2 elements; Period 2 Contains 8 elements; Period 3 contains 8 elements.
(ii) Hydrogen and Helium.
(iii) The atomic size of elements decreases on moving from left to right in Period.
Question 16: (i) What is the common feature of the electronic configurations of the elements at the end of Period 2 and Period 3?
(ii) If an element is in Group 7 (or Group 7A) is it likely to be metallic or non-metallic character?
(iii) Supply the missing word from those in brackets.
If an element has one electron in its outermost energy level (shell) then it is likely to be (metallic/non-metallic)
Answer: (i) The atoms of the elements at the end of period 2 and Period 3 contains 8 electrons in their outermost shell.
(ii) Non-metallic.
iii) Metallic
Question 17: An element Z has atomic number 16. Answer the following questions on Z:
(i) State the period and group to which Z belongs.
(ii) Is Z a metal or a non-metal?
(iii) State the formula between Z and Hydrogen.
(iv) What kind of a compound is this?
Answer: (i) 3rd period, 16th group.
(ii) Z is a non-metal.
(iii) H2Z
(iv) Covalent compound.
Question 18: What is similar in the electronic structure of Li, Na and K?
Answer: The electronic configuration of Li = 2, 1.
The electronic configuration of Na = 2, 8, 1.
The electronic configuration of K = 2, 8, 8, 1.
All the three have one electron in the outermost shell which they lose to form positively charged species, i.e., Li+, Na+ and K+.
Question 19: Answer the following questions regarding group 17 i.e., halogens of the periodic table.
(i) What are halogens?
(ii) Which group and sub-group are they placed in?
(iii) What is their valency?
(iv) Why are they called halogens?
(v) Why do they not occur free in nature?
Answer: (i) Halogens are the members of VII A group which have seven electrons in the outermost shell.
(ii) They are placed in group VII and sub-group A.
(iii) Their valency is —1.
(iv) They are called halogens because chlorine which is a halogen, in the form of sodium chloride is the most abundant salt in nature. Halogen means salt producer.
(v) They do not occur free in nature because they all are very reactive as they need just one electron to complete their outermost shell and every member of the halogen group tries to attain stable electronic configuration.
Question 20: State the factors which affect or influence the atomic size of the elements in a periodic table.
Answer: These are:
(i) Magnitude of nuclear charge
(ii) Number of shells
(iii) Screening or shielding effect
Question 21: In group 1 of the Periodic Table, three elements X, Y and Z have ionic radii 1.33Å, 0.95Å and 0.60Å respectively. Giving a reason, arrange them in the order of increasing atomic numbers in the group.
Answer: X – 1.33 Å; Y – 0.95 Å; Z – 0.60 Å.
The order of increasing atomic numbers is : Z < Y < X.
This order is due to the fact, that greater is the ionic radii, greater is the atomic number of the element. As the ionic radii increases, the number of electrons increases, i.e., the atomic number increases.
Question 22: (i) A boy has reported the radii of Cu, Cu+ and Cu2+ as 0.96Å, 1.22Å and 0.72Å respectively. However, it has been noticed that he interchanged the values by mistake. Assign correct values to different species.
(ii) Account for the difference in size of Na+ (0.96Å) and Mg2+ (0.65Å) both of which have the same number of electrons.
Answer: (i) Cu = 1.22Å; Cu+ = 0.96Å; Cu2+ = 0.72Å.
(ii) Mg2+ (0.65Å) is smaller than Na+ (0.96Å) because as the effective nuclear charge per electron increases, the electrons are more strongly attracted and pulled towards the nucleus which causes a decrease in size of the Mg2+ ion.
Question 23: What do you know about metallic character of an element ?
Answer: It is the character of an element to have no more than three electrons in its outermost shell and its ability to form positive ions by loosing these electrons.
Question 24: What is shielding effect or screening effect ? How does it govern the ionization energy of an atom ?
Answer: When the number of inner electrons is greater, then they shield the outermost electron from the nucleus in such a way that the outermost electron becomes free from any nuclear attraction. This is called the shielding or screening effect.
The shielding effect makes the electron very less firmly held by the nucleus on descending a group, so the ionisation energy decreases down a group.
Question 25: A group of elements in the Periodic Table are given below (Boron is first member of the group and Thallium is the last).
Boron
Aluminium
Gallium
Indium
Thallium
Answer the following questions in relation to the above group of elements : (i) Which element has the most metallic character ?
(ii) Which element wbuld be expected to have the highest electro-negativity ?
(iii) If the electronic configuration of Aluminium is 2, 8, 3, how many electrons are there in the outer shell of Thallium ?
(iv) The atomic number of Boron is 5. Write the chemical formula of the compound formed when Boron reacts with Chlorine.
(v) Will the elements in the group to the right of this Boron group be more metallic or less metallic in character ? Justify your answer.
Answer: (i) Thallium (ii) Boron,
(iii) Three (iv) BCl3 and
(v) Less metallic. Metallic character decreases from left to right on the periodic table.
Question 26: Arrange the following elements as directed
(i) Ar, He, Ne (in increasing order of electron shells)
(ii) Li, F, C, O (in increasing order of electron affinity)
(iii) Cl, Mg, P, Na (in increasing order of atomic size)
(iv) Cl, Li, F, N (in increasing order of electronegativity)
(v) Cl, S, Al, Na (in increasing order of ionisation potential)
(vi) Increasing order of atomic size.
(vii) Increasing non-metallic character
(viii) Increasing ionisation potential.
(ix) Increasing electron affinity.
(x) Decreasing electronegativity.
Answer: (i) Increasing order of number of electron shells: He, Ne, Ar
(ii) Increasing order of electron affinity: Li, C, O, F
(iii) Increasing order of atomic size: Cl, P, Mg, Na
(iv) Increasing order of electronegativity : Li, N, Cl, F
(v) Increasing order of ionisation potential : Cl, S, Al. Na.
(vi) F>Cl>Br>I.
(vii) I<Br<Cl<F.
(viii) I<Br<Cl<F.
(ix) F<Cl<Br<l.
(x) F>Cl>Br>I.
Question 27: (I) Arrange I, Cl and Br in an increasing order of their atomic size.
(ii) Arrange the following in the increasing order of their ionisation potential?
Li, Be, B, C, N, O, F.
(iii) What are the following groups known as:
(a) Group I (b) Group VII (c) Group VIIIA,
(iv) What was the significance of the Dobereiner triads or what is the achievement of Dobereiner’s trinads?
(v) Arrange the following nine elements into three Doberiner’s triads. The atomic weights of some of these elemcnts are to the left of their symbols. Supply the atomic weights of other elements by calculation.
137Ba 80Br 40Ca 127I K Cl 7Li Sr 23Na
(vi) An element X belongs to 3rd period and group II of the Periodic Table. State:
(a) The number of valence electrons (b) Valency
(c) Metal or non-metal, and (d) Name of the element.
Answer: (i) Cl<Br<I.
(ii) Li<Be<B<C<N<O<F.
(iii) (a) Group I—Alkali metals (b) Group VII—Halogens
(c) Group VIII A—Noble gases.
(iv) This was the first attempt to relate the properties of the elements to their atomic weights.
(v)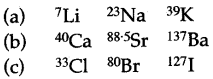
(vi) (a) 2 (b) +2 (c) Metal (d) Magnesium.
Question 28: How does the chemical reactivity of alkali metals and halogens vary ?
Answer: The reactivity of alkali metals increases as we move down the group. As the atomic number increases, the atomic racfcus also increases and the single electron in the valence shell is located further away from the nucleus. Hence, Li is least reactive and Francium is the most reactive. In case of halogens, the reactivity gradually decreases as we move down the group. All of them require one electron to complet, the outermost shell and the nuclear attraction is greater on the nearest shell (i.e., Ilnd shell in F) than in the fathest shell (i.e., vith shell in iodine). Hence, F is most reactive and I is least reactive.
Question 29: How do the following change, on going from left to right in a period of the Periodic Table ? Give example in support of your answer.
(i) Chemical reactivity of elements. (ii) Nature of oxides of the elements.
Answer: (i) The alkali metals are highly reactive. The chemical reactivity of elements decreases from left to right in a period of the Periodic Table, e.g., in the 3rd period, the order of chemical reactivity is:
Na> Mg >Al>Si>P>S>Cl
The order of chemical reactivity is the reverse of the order of electronegativity.
(ii) Oxides of elements in a particular period become less and basic and finally becomes acidic in character, e.g., oxides of third period: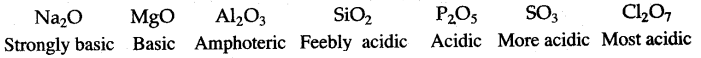
Question 30: Strongly basic Basic Amphoteric Feebly acidic Acidic More acidic Most acidic The elements of one short period of the Periodic Table are given below in order from left to right:
Li Be B C O F Ne
(i) To which period do these elements belong ?
(ii) One element of this period is missing. Which is the missing element and where should it be placed ?
(iii) Which one of the elements in this period shows the property of catenation ?
(iv) Place the three elements fluorine, beryllium and nitrogen in the order of increasing electronegativity.
(v) Which one of the above elements belongs to the halogen series ?
Answer: (i) 2nd period
(ii) Nitrogen. It should be placed between carbon and oxygen
(iii) Carbon
(iv) Beryllium < Nitrogen < Fluorine
(v) Fluorine
Question 31: (i) Electron affinities of two elements A and B are given as follows :
A = 3.79 electron volts and B = 3.56 electron volts
Which of them will ionize more easily and why ?
(ii) A, B and C are the elements or a Dobereiner’s triad. If the atomic mass of A is 7 and that of C is 39, what should be the atomic mass of B ?
(iii) Account for the difference in size of Fe3+ as Fe2+ = 0.76A and Fe3+ = 0.64Å.
Answer: (i) A will ionize more easily than B because more is the electron affinity of an element, the more will be the tendency to accept electrons and hence get ionized easily.
(ii)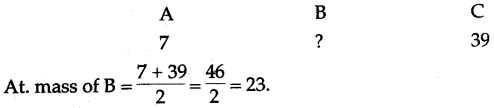
(iii) The size of Fe3+ (0.64Å) is smaller than the size of Fe2+ (0.76Å) because the effective nuclear charge per electron increases. The electron are more strongly attracted and pulled towards the nucleus. This causes decrease in the size of the Fe3+ ion.
Question 32: (i) For each of the following pairs, predict which one has greater ionization energy and greater electron affinity ?
(a) 1,1– (b) B, C (c) Li, Li+
(ii) Select the correct order of radii of three species : Ca, Ca+ and Ca2+:
(a) Ca > Ca+ > Ca2+ (b) Ca2+ > Ca+ > Ca
(c) Ca+ > Ca > Ca2+ (d) Ca+ > Ca2+ > Ca
Assign suitable reason.
Answer: (i)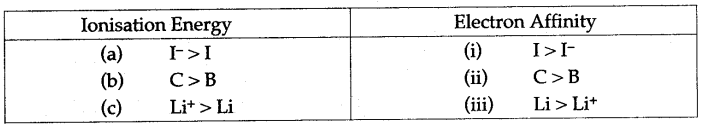
(ii) The correct order of radii is (a), i.e., Ca > Ca+ > Ca2+.
This is because due to the loss of electron, the effective nuclear charge per electron increases, the electrons are more strongly attracted and pulled towards the nucleus. This causes a decrease in the size of the positive ion.
Question 33: (i) The elements calcium, strontium and barium were put in one group or family on the basis of their similar properties:
(a) What are those similar properties?
(b) What is the usual name of the group or family?
(ii) Chlorine, bromine and iodine elements were put in one group on the basis of their similar properties:
(a) What are those similar properties?
(b) What is the common name of this group or family?
Answer: (i) (a) All the three have the same number of valence electrons in their outermost shell, i.e., 2. So they tare reactive, non-metallic, have low ionisation energy and low electron affinity.
(b) The name of this group is alkaline earth metals.
(ii) (a) All are non-metals and bad conductors of heat and electricity; diatomic in the gaseous state, form ionic compounds with non-metals.
(b) They are called halogen.
Question 34: Two different elements A and B have atomic number 12 and 14 respectively, write down:
(i) The electronic configuration of X and Y, using the notation 1s2,2s22p6…
(ii) The groups of Periodic Table to which they belong.
(iii) The principal oxidation states.
(iv) The formulae of their simplest chlorides.
(v) The explanation for the following:
The chloride of one is a good conductor of electricity in molten state while the other which is liquid, is not.
Answer: (i) The electronic configuration of A and B are:
A = 1s2,2s2 2p6, 3s2
B = 1s2, 2s2 2p6, 3s23p2
(ii) A belongs to Il’ and B belongs to IVth group of the Periodic Table.
(iii) The principal oxidation states of A and B are 2 and 4, respectively.
(iv) The formulae of their chlorides are ACl2 and BCl3, respectively.
(v) The chloride of A is an electrovalent compound and conducts electricity in molten state.
The chloride of B is a liquid covalent compound, hence is not a good conductor of electricity.
Question 35: (i) An element has an atomic number 16. State
(a) the period to which it belongs. (b) the number of valence electronš.
(c) whether it is a metal or non-metal.
(ii) Within a Group where would you expect to find the element with:
(a) The greatest metallic character? (b) The largest atomic size?
Answer: (1) (a) 3rd period (b) Six electrons (c) Non-metal
(ii) (a) At the bottom of the group. (b) At the bottom of the group.
Question 36: With reference to the first three periods of the modern periodic table, answer the questions given below:
(i) Write the formula of theslphate of the element with atomic number 13.
(ii) Name the clement which has the highest ionisation potential.
(iii) What features of the atomic structure accounts for the similarities in the chemical properties of the elements in group VIIA of the periodic table?
(iv) How many electrons arc present in the valence shell of the element with atomic number 18?
(v) What is the electronic configuration of the element in the third period which gains one electron to change into an anion?
(vi) What is the name given to the energy released when an atom in its isolated gaseous state accepts an electron to form an anion?
(vii) What type of bonding will be present in the oxide of the element with atomic number 1?
Answer: (i) Al2 (SO4)3
(ii) Helium
(iii) Seven electrons in their valence shells
(iv) Eight
(v) 2, 8, 7 (vi) Electron affinity
(vii) Covalent bonding
Figure/Table Based Questions
Question 1: Consider the section of the periodic table given below :
| Group Numbers | IA 1 | HA 2 | IIIA 13 | IVA 14 | VA 15 | VIA 16 | VIIA 17 | 0 18 |
| Li | D | O | J | Ne | ||||
| A | Mg | Y | Si | H | Y | |||
| B | C | F | G | L |
In this table B does not represent Boron
C does not represent Carbon
F does not represent Fluorine H does not represent Hydrogen
K does not represent Potassium
You must see the position of the element in the periodic table. Some elements are given in their own symbol and position in the periodic table. While others are shown with a latter. With reference to the table :
(i) Which is the most electronegative ?
(ii) How many valence electrons are present in G ?
(iii) Write the formula ofrthe compound between B and H.
Answer: (i) J (ii) Five (iii) B2H
Question 2: Given below is the part of Periodic Table:
| Li | Be | B | C | N | O | F |
| Na | Mg | Al | Si | P | s | Cl |
(i) How does metallic character of an element change as one moves from.
(a) Left to right in period ? (b) Top to bottom in group ?
(ii) How does the valency of elements change with respect to hydrogen as one moves from left to right in period ?
(iii) (a) What is the valency of element silicon ?
(b) Will it form a covalent or electrovalent bond with hydrogen ?
(iv) Which are the most metallic and the most non-metallic elements in above table.
Answer: (i) (a) As one moves from left to right in period, the metallic character of an element
decreases and then it changes to non-metallic character.
(b) As one moves from top to bottom in period, the metallic character of the elements increases.
(ii) The valency of elements with respect to hydrogen is stated as under:
I group +1
II group +2
III group +3
IVgroup -4
V group -3
VI group -2
VII group -1
(iii) (a) The valency of element silicon is -4
(b) Silicon will form a covalent compound with hydrogen.
(iv) Sodium is the most metallic arid chlorine is most non-metallic element.
Question 3: The diagram given below is a part of Periodic Table. Study the table and answer the questions given below the table :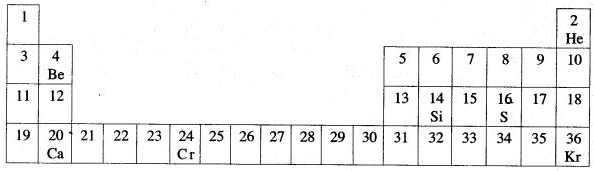
(i) Name two elements in same group of Periodic Table.
(ii) Name the Transition metal.
(iii) Name one elements, whose oxide has a very low melting point.
(iv) Name the dement, whose oxide is basic.
(v) Name an element, which reacts vigorously with water.
(vi) Name an element, which form basic oxide.
(vii) Which element shows variable oxidation state ?
(viii) Which element is used in semi conductors ?
(ix) Which elements posses complete valence shell ?
(x) Which element forms very corrosive acid ?
Answer: (i) Oxygen and sulphur (ii) Chromium
(iii) Calcium (iv) Sulphur
(v) Calcium (vi) Calcium
(vii) Chromium (viii) Silicon
(ix) Helium and Krypton
(x) Chromic acid produced by chromium.
Question 4: In the portion of the Periodic Table given below, the letters A, B, represent the elements in periods 2 and 3 and groups 1, 2,13,14,15,16,17 and 18 which are not die usual symbols of the elements.
| 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Period 2 | A | B | C | D | E | F | G | H |
| Period 3 | I | J | K | L | M | N | O | P |
| Q | R | S | T | U |
Study the table and answer the following questions :
(i) Which is the most electropositive element ?
(ii) Which is the most electronegative element ?
(iii) Which elements have properties that are similar to those of the element O ?
(iv) Which elements are the noble gases ?
(v) Which elements have a valency of 4 ?
(vi) Which is more metallic, Q or R ?
(vii) Which is more non-metallic, E or M ?
(viii) What is the collective name for the elements G, O, and S ?
(ix) Which elements are represented by the letters I, J, N, and O ?
Answer: (i) T, (ii) G, (iii) G and S, (iv) H and P, (v) D and L, (vi) Q, (vii) E, (viii) The halogens, (ix) I : Sodium, J : Magnesium; N: Phosphorus; O: Chlorine.
Question 5: The electronegativities (according to pauling) of the elements in period 3 of the portion of Periodic Table are as follows when the elements arranged in alphabetical order :
| Al | Cl | Mg | Na | P | S | Si |
| 1.5 | 3.0 | 1.2 | 0.9 | 2.1 | 2.5 | 1.8 |
(i) Arrange the elements in the order in which they occur in the Periodic Table from left to right.
(The group 1 element first, followed by the group 2 element and so on, up-to group 7)
(ii) The element below sodium in the same group would be expected to have a ………….. (lower/higher) electronegativity than sodium and the element above chlorine would be expected to have a ……….. (lower/higher) ionization potential than chlorine.
Answer: (i) Arrangement of elements in the III rd group of Periodic Table is given below :
Na, Mg, Al, Si, P, S, Cl.
(ii) Lower, Higher.
Question 6: The questions refers to the elements of the Periodic Table with atomic numbers from 3 to 18. Some of the elements are shown by letters, but letters are not usual symbols of the elements:
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P |
Which of these:
(i) Is a noble gas ? (ii) Is a halogen ?
(iii) Is an alkali metal ? (iv) Is an element with valency ?
Answer: (i) P is a noble gas with atomic number 18, i.e., 2, 8, 8.
H is a also a noble gas with atomic number 10, i.e. 2, 8.
(ii) G is halogen with atomic number 9, i.e. 2, 7.
O is also a halogen with atomic number 17, i.e. 2,8, 7.
(iii) A and I are alkali metals.
(iv) D and L are elements with valency 4.
Question 7:
| Group number | IA 1 | HA 2 | IIIA 13 | IVA 14 | VA 15 | VIA 16 | VIIA 17 | 0 18 |
| 2nd period | Li | D | O | J | Ne | |||
| A | Mg | E | Si | H | M | |||
| R | T | I | Q | u | y |
- In this table H does not represent hydrogen.
- Some elements are given in their own symbol and position in the periodic table.
- While others are shown with a letter.
With reference to the table answer the following questions :
(i) Identify the most electronegative element.
(ii) Identify the most reactive element of group 1.
(iii) Identify the element from period 3 with least atomic size.
(iv) How many valence electrons are present in Q ?
(v) Which element from group 2 Would have the least ionization energy ?
(vi) Identify the noble gas of the fourth period.
(vii) In the compound between A and H what type of bond would be formed and give the molecular formula for the same.
Answer: (i) J (ii) R (iii) M
(iv) Five (v) T (vi) y-Krypton
(vii) Ionic bond. Molecular formula ⟶ A2H
Question 8: Study the table above and answer the following questions carefully:
| Elements | A | B | C |
| Mass number | 23 | 20 | 35 |
| Number of neutrons | 12 | 10 | 18 |
(i) Write the atomic number and electronic configuration of elements A, B and C.
(ii) To which groups do A.. B and C belong?
(iii) To which periods do A, B and C belong?
(iv) Which anìongst A, B and C is (1) an alkali metal (ii) noble gas (iii) halogen?
Answer: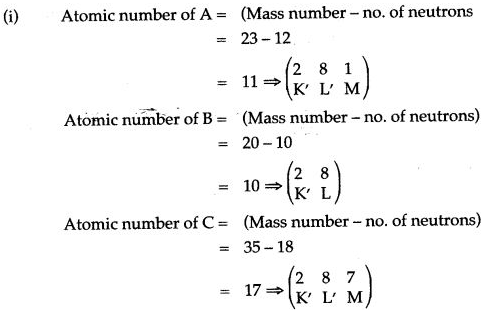
(ii) A belong to IA group, B belong to zero group, C belongs to VIIA group, Criterion: no. of valence electrons.
(iii)A belongs to 3rd period, B belongs to 2nd period, C belongs to 3rd period, Criterion => no. of shells.
(iii) A is an alkali metal, B is a noble gas, C is a halogen.
Reasoning Based Questions
Question 1: Explain why the elements placed in the same group of the Periodic Table have the same chemical properties ?
Answer: It is because they have the same number of valence electrons in the outermost shell of their atoms.
Question 2: Why group IA elements are called Alkali metals ?
Answer: Group IA elements are called alkali metals because their hydroxides are soluble in water and form strong bases.
Question 3: Why sodium is a metal while sulphur is a non-metal ?
Answer: Sodium has a larger atomic radii and lower ionization potential than sulphur. Hence sodium is a metal while sulphur is a non metal.
Question 4: Alkali metals are good reducing agents.
Answer: Alkali metals are good reducing agents because alkali metals have one valence electron which they lose to attain stability. Hence, they themselves undergo oxidation causing reduction of others and are good reducing agents.
Question 5: Why are the elements sodium and chlorine in the same period of the Periodic Table ?
Answer: Because the atoms of both the elements have three shells containing the electrons.
Question 6: Sodium atom, Na forms the positive ion Na+, but chlorine atom Cl, does not form the positive Cl+ ion.
Answer: Sodium is the first element of period 3 whereas chlorine is the last but one element of the same period. Since ionization potential increases across a period, the ionization potential of Na is much smaller than that of Cl. Hence, Na can lose an electron to form Na+ ion whereas Cl cannot lose an electron to form Cl+ ion.
Question 7: Potassium atom is larger than sodium atom. Why ?
Answer: Potassium is placed below sodium in group 1. It, therefore has one more electron shell. Na atom has three electron shells (2, 8,1); K atom has four (2, 8, 8,1). So, potassium atom is bigger than sodium atom.
Question 8: Magnesium atom is smaller than calcium atom. Why ?
Answer: Magnesium atom precedes calcium atom in the same group, i.e., group 2. Magnesium atom has got three electron shells (2, 8, 2) whereas calcium atom has four electron shells (2, 8, 8, 2). So, calcium atom is larger than the sodium atom.
Question 9: Magnesium atom is smaller than sodium atom. Why ?
Answer: Magnesium come after sodium in the same period. Atoms of both elements have three electron shells (Na : 2, 8, 1 : Mg : 2, 8, 2). But the nuclear charge of sodium is + 11 and that of magnesium is + 12. Hence, the electron shells are pulled inward more strongly in Mg atom than in Na atom. Hence, Mg atom is smaller than Na atom.
Question 10: Which is larger Na+ or K+ ? Why ?
Answer: K+ is larger than Na+ because the ionic radius increases in a particular group on moving from top to bottom due to increase in the principle energy shell though the number or electrons in the valence shell remain the same.
Question 11: Mg2+ ions is smaller than O2- ion although both are iso-electronic. Explain.
Answer: Mg2+ ion is smaller than O2- ion though both are iso-electronic. The nuclear charge in Mg2+ is + 12 and O2- is + 8, so with the increase in nuclear charge the size decreases and, hence O2- > Mg2+.
Question 12: Why the atomic size decreases in a period as we move from left to right ?
Answer: As we move from left to right across a period, the number of shells remain the same. As the atomic number increases, the nuclear charge increases and there is a greater attraction between the nucleus and the electrons. The atomic size, therefore, decreases across a period.
Question 13: The reducing power of elements increases as one goes down a group ?
Answer: When an atom loses an electron then the element is said to be a reducing agent. The reducing power of an element depends upon how quickly it can lose electrons. In case of electrons held very loosely by the nucleus such element can easily lose their valence electrons and hence, higher reducing agent.
Question 14: The reducing power of elements decreases as on one move from left to right in a period ?
Answer: The reducing power of an element depends upon, how quickly it can lose electrons in the valence shell. As one moves from left to right in Periodic Table, the electrons in valence shell are held more tightly because of increase of nuclear charge. Thus, the tendency of atoms to lose their electrons gradually decreases and so does the reducing power.
Question 15: Why the oxidising power of elements increases on moving from left to right along a period in the periodic table ?
Answer: Because on moving from left to right along a period in the periodic table the electron affinity of elements increases.
Question 16: Why ionization potential of the element increases across a period ?
Answer: Ionization potential of an element is the amount of energy required to remove one or more electrons from the outermost shell of an isolated gaseous atom.
Across a period, the atomic radii decreases due to increase in nuclear charge due to addition of electrons which results in greated attraction of valence shell electrons. Hence, I.P. increases.
Question 17: Why the second ionization energy of an element is greater than its first ionization energy ?
Answer: More energy is required to remove an electron as it holds more firmly by the unipositive ion.
Question 18: Why is ionisation energy of O less than that of N ?
Answer: Ionisation energy of O is less than that of N because it is very easy to remove electrons from oxygen than from nitrogen. Hence, ionisation energy of O is less than that of N.
Question 19: Noble gases have zero electron affinity values.
Answer: Noble gases have zero electron affinity values because they have stable electronic configuration and have no tendency to take an additional electron. Hence, no energy is released and their electron affinity is zero.
Question 20: Why elements with low ionization potential exhibit metallic properties ?
Answer: Metallic character increases with decrease in ionization potential. Lower the value of ionization potential, the greater is the tendency of an atom to lose electrons.
Comments
Post a Comment