Study Of Compounds: Hydrochloric Acid
Short Questions
Question 1: (i) State one condition under which chlorine and hydrogen react to form hydrogen chloride gas.
(ii) Give balanced chemical equation for the above reaction.
(iii) Name the gas which is a covalent compound but becomes electrovalent when dissolved in water ?
(iv) For which gas, ammonia fountain experiment can be used ?
Answer: (i) Presence of diffused sunlight.
(ii) H2 + Cl2 ⟶ 2HCl
(iii) Hydrogen chloride (HCl) gas.
(iv) Hydrogen chloride gas.
Question 2: A colourless gas G fumes strongly in the air. The gas gives dense white fumes when a glass rod dipped in ammonia solution is held near the gas.
Answer the following questions:
(i) Name the gas G.
(ii) Name two chemicals used in the preparation of the gas G.
(iii) Write the chemical equations for the reaction of the chemicals named in (ii) when :
(a) The reaction mixture is not heated.
(b) The reaction mixture is heated above 100°C.
(iv) Why does the gas G fume strongly in air ?
(v) Why does the gas G form dense white fumes with ammonium hydroxide ?
Answer: (i) The gas G is hydrogen chlorine gas.
(ii) The chemicals are (i) sodium chloride, (ii) concentrate sulphuric acid.
(iii) (a) NaCl + H2SO4 (conc.) ⟶ NaHSO4 + HCl (g)
(b) Nacl + NaHSO4 ⟶ Na2SO4 + HCl (g)
(iv) It is because the HCl gas is extremely soluble in water. Thus the gas dissolves in water vapour present in the air to form tiny droplets of hydrochloric acid, which appear in the form of fumes.
(v) The HCl gas reacts with vapours of ammonium hydroxide to form very fine solid particles of ammonium hydroxide which are white in colour. These white particles of solid ammonium hydroxide appear in the form of white fumes.
Question 3: (i) How will you dry HCl acid gas.
(ii) Give three tests of hydrogen chloride.
(iii) Which two colourless gases combine to form a white solid.
Answer: (i) HCl gas can be dried by passing it over conc. H2SO4, which acts as a powerful dehydrating agent.
(ii) Tests for Hydrogen Chloride.
(1) It gives dense white fumes with a rod dipped in NH4OH solution.
(2) It produces white ppt. with AgNO3 solution.![]()
(3) It turns moist blue litmus red.
(iii) NH3 and HCl gases combine to form a white solid NH4Cl![]()
Question 4: (i) (a) What must be added to sodium chloride to obtain hydrogen chloride ?
(b) Write the equation for the reaction which takes place in (a) (i) above.
(c) What would you see when hydrogen chloride is mixed with ammonia ?
(ii) Hydrogen chloride dissolve in water forming an acidic solution:
(a) Name the experiment which demonstrates that hydrogen chloride is very soluble in water.
(b) Give three distinct tests (apart from using an indicator) you would carry out with this solution to illustrate the typical properties of an acid.
Answer: (i) (a) Concentrated Sulphuric acid.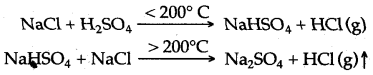
(c) When aqueous solution of ammonia is taken in the jar of hydrogen chloride, it forms dense white fumes of ammonium chloride.
NH3 + HCl ⟶ NH3Cl
(ii) (a) Fountain experiment.
(b) An acid reacts with:
(I) Metal carbonates and bicarbonates with effervescence to liberate CO2.
(II) Acids react with metal sulphides to liberate H2S gas which has smell of rotten eggs
(III) Acids react with metal sulphites to liberate SO2 gas.
Question 5: (i) (a) Name the oxidising agent in the reaction between Manganese dioxide and cone, hydro-chloric acid.
(b) State your observation when a rod dipped in ammonium hydroxide solution is brought near a gas jar containing hydrogen chloride gas.
Manganese (IV) oxide, lead (IV) oxide and red lead (Pb3O4) react with concentrated hydrochloric acid liberating chlorine.
(ii) (a) What is the common property being shown by these metal oxides ?
(b) Write the equation for the reaction of concentrated hydrochloric add with Pb3O4.
(c) What kind of compound can be added to bleaching powder to obtain chlorine ?
Answer: (i) (a) Manganese dioxide acts as an oxidising agent.
(b) Dense white fumes appear in the jar on account of formation of fine particles of ammonium chloride which get suspended in the gas.
(ii) (a) Oxidizing agents
(b) Pb3O4 + 8HCl ⟶ 3PbCl2 + 4H2O + Cl2
(c) Dilute acid (Hydrochloric acid)
Question 6: Answer the following questions, stating your answer only to compounds in the following list: Silver nitrate, hydrochloric acid, chlorine, ammonia, bleaching powder.
(i) What is water sterilizer ?
(ii) Which compound forms curdy white precipitate with hydrogen chloride ?
(iii) Name the gas which produces dense white fumes with ammonia, write the balanced chemical equation.
Answer: (i) Chlorine is water sterilizer.
(ii) Silver nitrate and hydrochloric acid forms white ppt.
AgNO3 + HCl ⟶ AgCl (White ppt.) + HNO3
White ppt.
(iii) Hydrogen chloride (HCl)
NH3 + HCl ⟶ NH4Cl (Dense white fumes)
Question 7: (i) When moist chlorine reacts with hydrogen sulphide, two products are formed :
(a) A gas which fumes in moist air; and
(b) A yellow solid.
Name these products.
(ii) What type of reaction is taking place when chlorine acts as a bleaching agent ?
Answer: (i) (a) Hydrogen chloride gas (b) Sulphur
(ii) Oxidation reaction.
Question 8: From the gases-ammonia, hydrogen chloride, hydrogen sulphide, sulphur dioxide-Select the following:
(i) The gas which gives a white percipitate when reacted with silver nitrate solution
acidified with dilute nitric acid.
(ii) A solution of hydrogen chloride in water is prepared. The following substances are added to separate portions of the solution.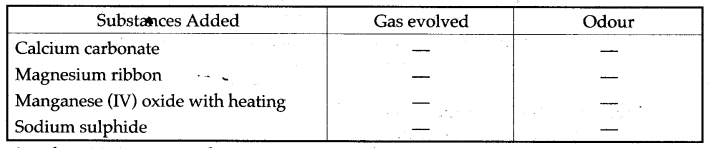
Complete the table by writing the gas evolved in each case and its odour, (i) Hydrogen chloride
Answer: (i) hydrogen chloride
(ii)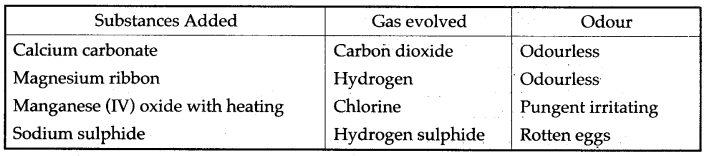
Question 9: What is aqua regia ? How does it help in dissolving Gold or Platinum.
Answer: A mixture of 1 part of cone, nitric acid and 3 parts of cone, hydro chloric acid by weight is called aqua regia.
The cone. HCl and conc. HNO3 reacts to form hascent chlorine which reacts with Gold or Platinum to form their respective soluble chlorides.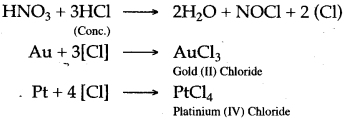
Question 10: State three uses of hydrochloric acid.
Answer: (i) It is used in the manufacture of silver chloride, which is used widely in photography.
(ii) It is used in the manufacture of dyes, drugs and paints.
(iii) It is used for cleaning metal surface before painting, electroplating, galvanising, soldering etc.
Figure/Table Based Questions
Question 1: Refer to the flow chart diagram below and give balanced equations with conditions, if any, for the following conversions A to D.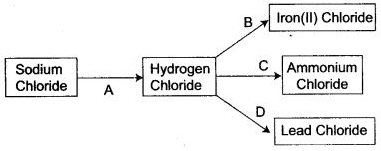
Answer: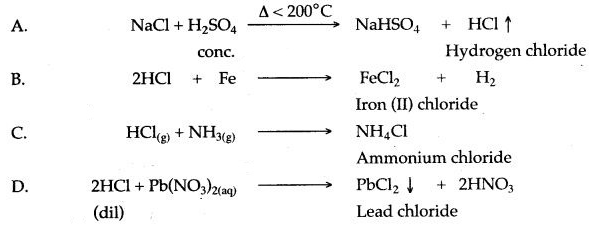
Question 2: In the laboratory preparation of hydrochloric acid, HCl gas is dissolved in water.
(i) Draw a diagram to show the arrangement used for the absorption of HCl in water.
(ii) Why is such an arrangement necessary ? Give two reasons.
(iii) Write the chemical equations for the laboratory preparation of HCl gas when the reactants are :
(A) below 200°C (B) above 200°C
Answer: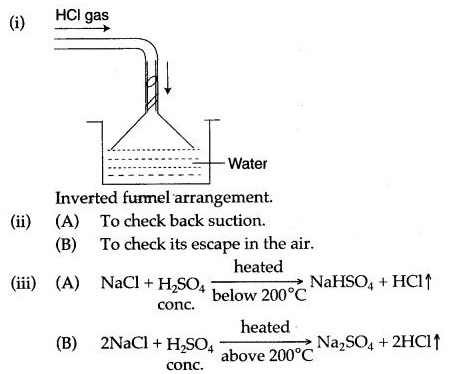
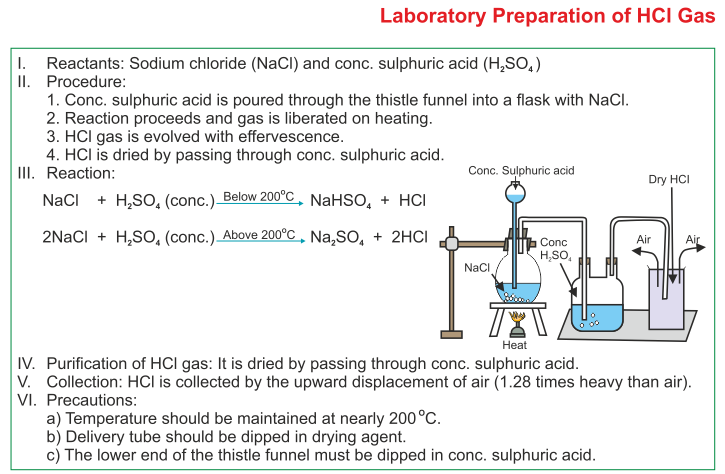
Question 3: The diagram shows an apparatus for the laboratory preparation of hydrogen chloride.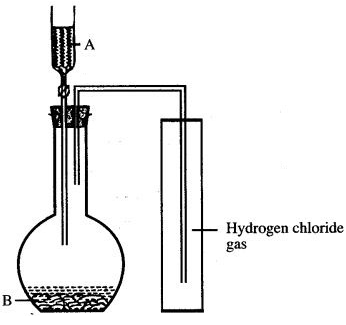
(i) Identify A and B.
(ii) Write the equation for the reaction.
(iii) How would you check whether or not the gas jar is filled with hydrogen chloride ?
(iv) What does the method of collection tell you about the density of hydrogen chloride ?
Answer: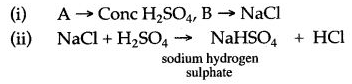
(iii) If a moist blue litmus brought near the mouth of gas jar turns red, the gas jar is filled with HCI.
(iv) Hydrogen chloride is denser than air.
Question 4: Study the figure given below and answer the questions that follow: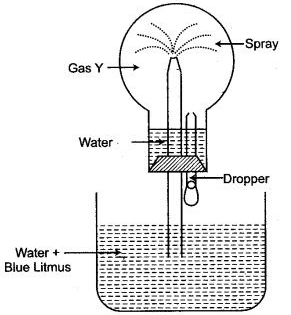
(i) Identify the gas Y.
(ii) What property of gas Y does this experiment demonstrate ?
(iii) Name another gas which has the same property and can be demonstrated through this experiment.
Answer: (i) Y is hydrochloride (HCl) gas.
(ii) Gas Y is highly soluble in water.
(iii) Ammonia gas.
Question 5: The given figure shown is for the preparation of an acid.
(i) Name the acid prepared by this method.
(ii) Name the reactants used.
(iii) Why an empty flask is used.
(iv) What is the drying agent used ? Why is this drying agent chosen.
(v) What is the role of inverted funnel in the arrangement ?
Answer: (i) Hydrochloric acid.
(ii) Sodium chloride and cone, sulphuric acid.
(iii) An empty flask is used to prevent back suction. If back susetin occurs, the water will collect in it and will not reach the generating flask.
(iv) The drying agent used is concentrated sulphuric acid. Because it does not react with hydrogen chloride.
(v) The role of inverted funnel in the arrangement is :
(a) Prevents back-suction of water.
(b) Providesa large surface area for absorption of HCl gas.
Question 6: (i) Name the experimental illustrated in the diagram.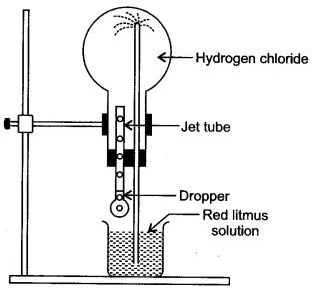
(ii) Which property of hydrogen chloride is demonstrated by this experiment.
(iii) State the colour of the water that has entered the round bottomed flask.
Answer: (i) Fountain experiment.
(ii) Hydrogen chloride (HCl) is highly soluble in water.
(iii) The colour of water that has entered the round bottomed flask is red.
Reasoning based Questions
Question 1: Mixture of sodium chloride and concentrated sulphuric acid does not heated above the temperature of 170°C while preparing hydrogen chloride. Why ?
Answer: The mixture of sodium chloride and concentrated sulphuric acid is not heated above 170°C in preparing hydrogen chloride gas because at a higher temperature sodium sulphate is formed which is a hard substance and difficult to remove from the reaction flask.
2NaCl + H2S04 → Na2S04 + 2HCl
Question 2: Hydrogen chloride gas cannot be dried over quick time. Why ?
Answer: Because quick lime is basic in nature and combines with moist hydrogen chloride gas forming calcium chloride.
Question 3: Quick lime and phosphorus pentaoxide cannot be used for drying hydrochloric acid gas. Why?
Answer: Quick linie and phosphorus pentaoxide cannot be used for drying HCl gas, because both reacts with HCl.
Question 4: Hydrogen chloride is not collected over water. Why ?
Answer: Hydrogen chloride is not collected over water because it is highly soluble in water.
Question 5: When the stopper of a bottle full of hydrogen chloride gas is opened there are fumes in the air ?
Answer: This is because hydrogen chloride gas has an affinity for water, hence, when the stopper is opened it immediately reacts with water vapour present in the atmosphere which leads to the formation of fumes.
Question 6: Dilute hydrochloric acid cannot be concentrated by distilling (boiling) the dilute acid. Why ?
Answer: When dilute hydrochloric acid is distilled, a constant boiling mixture containing 20 – 24% of hydrochloric acid distills over unchanged at 760 mm Hg pressure. This constant boiling mixture cannot be separated into its constituents by simply distilling.
Question 7: Anhydrous HCl is a poor conductor while aqueous HCl is an excellent conductor. Why ?
Answer: This is because anhydrous HCl does not contain any free ions. But when HCl is dissolved in water, it dissociates into hydronium ion (H3O+) and chloride ion (Cl–). Due to the presence of free ions, aqueous solution of HCl conducts electricity.
Question 8: Sodium is not used to prepare hydrogen from hydrochloric acid (or any other acid). Why ?
Answer:
Sodium is not used to prepare hydrogen from acids because sodium metal is highly reactive. So, the reaction with acids is much exothermic and there are more chances of explosion. It is also very dangerous to handle sodium metal.
Question 9: Silver nitrate crystals are dissolved in distilled water and not in tap water in order to prepare a solution of silver nitrate as a laboratory reagent. Why ?
Answer: Tap water always contains some amount of dissolved sodium chloride. Thus when the solution of silver nitrate is prepared in tap water, it reacts to form curdy white precipitate of silver chloride.
AgNO3 + NaCl → AgCl + NaNO3
To prevent the above chemical reaction, the solution of silver nitrate is prepared in distilled water.
Question 10: Water for drinking purpose and in swimming pools, is treated with chlorine. Give reason.
Answer: Water for drinking purpose and in swimming pools is treated with chlorine because it sterilises the water. Due to its strong oxidizing action, it destroys bacteria, fungus and other microorganisms.
Question 11: Why is it inadvisable to use bleaching powder as a disinfectant when it is mixed with acids ?
Answer: When an acid is mixed into bleaching powder, chlorine gas is liberated which pollutes the atmosphere and makes it unsafe for breathing.
CaOCl2 + H2SO4 → CaSO4 + H2O + Cl2
Chemical Tests
Question:
1. Manganese dioxide and copper (II) oxide.
2. Hydrogen chloride gas and carbon dioxide gas.
3. Give three tests for HCl gas.
Answer: 1. When cone, hydrogen chloride is added to manganese dioxide, greenish yellow gas (Cl2) is liberated.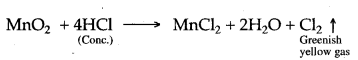
When cone, hydrogen chloride is added to copper (II) oxide, no. gas is liberated but the solution turns bluish because of the formation of copper chloride.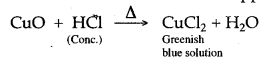
2. When passed into silver nitrate solution, forms a curdy white precipitate of silver chloride.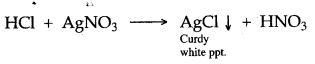
When passed into lime water, forms a milky white precipitate of calcium carbonate.![]()
3.(i) When a glass rod dipped in ammonia, solution is held near the vapours of the acid, it form a dense white fumes of ammonium chloride.
(ii) When hydrochloric acid is treated with silver nitrate solution, it forms curdy white precipitate which is soluble in excess of ammonium hydroxide solution.
(iii) When hydrochloric acid is boiled with manganese dioxide, greenish yellow chlorine gas is evolved.
Balancing/Writing the Chemical Equations
Question 1: Write balanced equations for the reaction of dilute hydrochloric acid with each of the following :
| 1. | Iron | 2. | Sodium hydrogen carbonate |
| 3. | Iron (II) sulphide | 4. | Sodium sulphite |
| 5. | Sodium thiosulphate solution | 6. | Calcium bicarbonate |
| 7. | Calcium carbonate | 8. | Sodium hydroxide |
| 9. | Zinc metal | 10. | Potassium permanganate |
| 11. | Red lead heated | 12. | Magnesium metal |
| 13. | Ammonium hydroxide. | 14. | Magnesium sulphite. |
| 15. | Sodium hydrogen sulphide. | 16. | Manganese dioxide. |
Answer: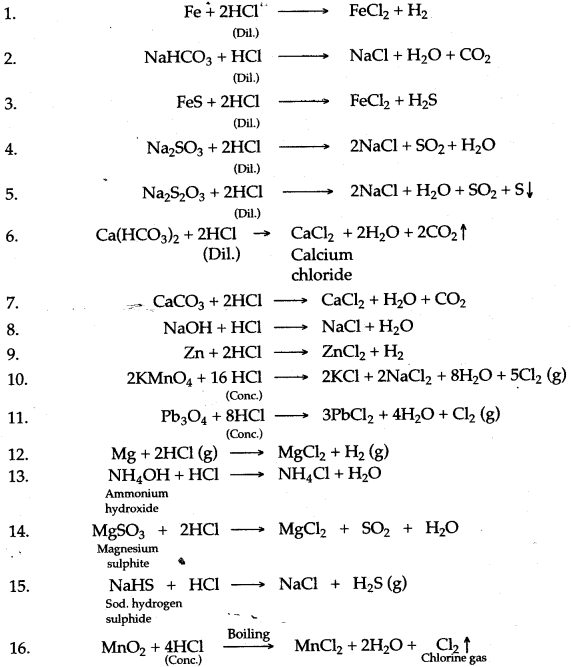
Question 2: Write balanced equation for the reaction of hydrochloric acid with each of the following :
1. Marble chips
2. Calcium sulphite
3. Lead nitrate solution.
4. Mangnese oxide.
Answer: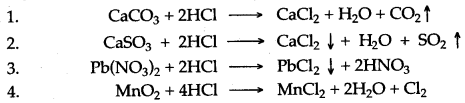
Question 3: Complete and balance the following equations :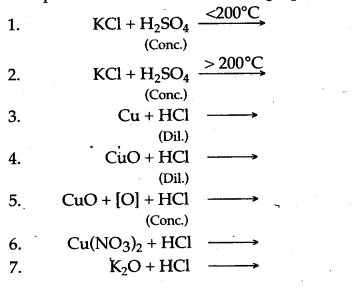
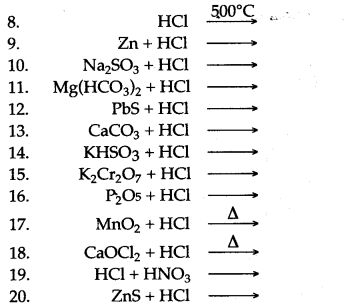
Answer: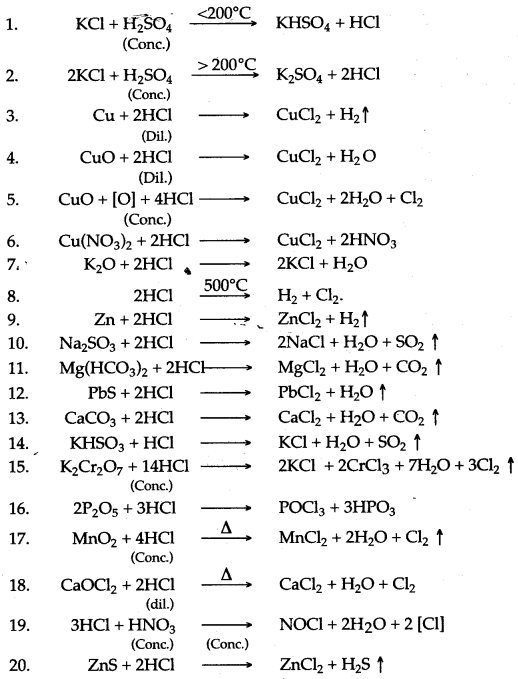
Comments
Post a Comment