Mole Concept
Short Questions
Question 1: State Gay-Lussac’s Law of combining volumes.
Answer: The law states that—Under same conditions of temperature and pressure, the volume of gases taking part in a chemical reaction show simple whole number ratio to one another and to those of products if gaseous.
Question 2: (i) When gases react together, their reaction volume bears a simple ratio to each other, under the same conditions of temperature and pressure. Who proposed this gas law ?
(ii) What is the volume (measured in dm3 or litres occupied by one mole of gas at S.T.P. ?
Answer: (i) This law was proposed by “Gay Lussac”.
(ii) One mole of gas occupies 22.4 litre at S.T.P.
Question 3: Under the same conditions of temperature and pressure, one collects 2.2 litre of CO2, 3.3 litres of Cl2, 5.5 litres of hydrogen, 4.4 litres of nitrogen and 1.1 litres of SO2. In which gas sample their will be:
(i) Greatest number of molecules. (ii) The least number of molecules.
Answer: We know that 22.4 litres of any gas at S.T.P. has 6.023 × 1023 molecules. If the volume of gas at S.T.P. is more than 22.4 litres, then the number of molecules will be greater and vice-versa.
(i) 5.5 litres of hydrogen will contain greatest number of molecules.
(ii) 11 litres of sulphur dioxide will contain least number of molecules.
Question 4: Under the same conditions of temperature and pressure, you collect 2 litres of carbon dioxide, 3 litres of chlorine, 5 litres of hydrogen, 4 litres of nitrogen and 1 litre of sulphur dioxide. In which gas will there be sample ?
(i) The greatest number of molecules (ii) The least number of molecules. Justify your answer.
Answer: The greatest number of molecules will be in 5 litres of hydrogen and the least number of molecules in 1 litre of sulphur dioxide. The justification is based on Avogadro’s law, which states that equal volumes of all gases, under conditions of same temperature and pressure, contain same number of molecules. So greater the volume, greater will be the number of molecules.
Question 5: How does Avogadro’s law explain Gay-Lussac’s law of gaseous volumes ?
Answer: Avagadro’s law states that equal volumes of all gases contain equal number of molecules under similar conditions of temperature and presure. Since, when gases react chemically, they do so in volumes which bear a simple whole number ratio to each other and to the volume of products, provided the products are also in gasesous state under similar conditions of temperature and pressure. This is what Gay Lussac’s law states. For example, in the reaction of carbon monoxide with oxygen, two volumes of carbon monoxide react with one volume of oxygen to give two volumes of carbon dioxide under similar conditions of temperature and pressure.
2CO + O2 —> 2 CO2
2 vol. 1 vol. 2 vol.
The volume ratio of carbon monoxide, oxygen and carbon dioxide is 2 : 1 : 2.
Question 6: What is the relationship between gram molecular weight and gram molecular volume at S.T.P. ?
Answer: Density of gas is defined as mass per unit volume. Volume is usually taken as 1 dm3 at S.T.P.
Molar volume of hydrogen:
Density of hydrogen = 0 09 gm/dm3 at S.T.P.
Gram molecular weight of hydrogen = 2.016 g 0.09 g of hydrogen occupies volume = 1 dm3 at S.T.P.
2.016 g of hydrogen occupies volume = dm3 at S.T.P.
= 22.4 dm3 at S.T.P.
As 2.016 g of hydrogen = 1 gram molecular weight
∴ 1 gram molecular weight of hydrogen occupies 22.4 dm3 at S.T.P.
Question 7: (i) What is ‘mole scale’ of a compound ?
(ii) What is the relation between atomic mass and equivalent mass ?
Answer: (i) The molecular weight of a compound expressed in gram is known as “a mole” of a compound. The multiples of the fractions of a mole give different mole values of a compound. The molecular weight of carbon dioxide is 44, hence 44 gm is one mole of carbon dioxide. 88 gm, and 176 gm of carbon dioxide represent 2 moles and 4 moles respectively.
In general,
(ii) Atomic mass = Equivalent mass × Valency
Question 8: Explain the following :
(i) Is it possible to change the temperature and pressure of a fixed mass of gas without changing its volume ?
(ii) One mole of hydrogen contains 2 × 6.023 × 1023 atoms of hydrogen where as one mole of helium contains 6.023 × 1023 atoms of helium.
Answer: (i) Yes, an increase in temperature produce an increase in volume which can be reduced (changed) to original volume by the increase in pressure.
(ii) Hydrogen is a diatomic gas.
So one molecule of hydrogen = 2 atoms
∴ 1 mole or 6.023 × 1023 molecules of H2 = 2 × 6.023 × 1023 atoms
On the other hand, helium is monoatomic gas,
∴ One molecule of helium 1 atom of He
or 6.023 × 1023 molecules of He = 6.023 × 1023 atoms of He.
Question 9: How will you differentiate between atomic weight and actual weight of an element ?
Answer:
| Atomic weight | Actual weight |
| (i) It is the number of times an atom of an element is heavier than a carbon atom (C12). | It is the weight of an atom as compared to a standard weight of lg. |
| (ii) Its numerical value is quite large and is conveniently used for comparing the weights of various atoms and making calculations. | Its numerical value is quite small and is inconvenient to use in comparing the weights of various atoms and making calculations. |
| (iii) It represents the mass of 6.023 x 1023 atoms. | It represents the mass of one atom. |
| (iv) It is simply a number as it expresses the ratio between two weights. | It is the absolute weight of an atom and is generally expressed in grams. |
Question 10: What do you understand by the statement “The vapour density of CO2 is 22″ ? The molecular weight of the gas is twice its vapour density.
Answer: Molecular weight = 2 × molecular weight of carbon dioxide
CO2 = 12 + 2 (16) = 12+ 32 = 44 gram.
The vapour density of carbon dioxide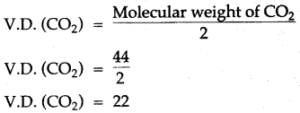
The vapour density of CO2 is 22 indicates that the mass of x litres of hydrogen gas or the molecular mass of CO2 is 44 gm.
thus, the vapour density of carbon dioxide (CO2) is 22.
Question 11: What are the applications of Avogadro’s law ?
Answer: (i) It determines the molecule formula of a gas.
(ii) It determines atomicity of gases.
(iii) It explains Gay-Lussac’s law of combining volumes.
(iv) It establishes the relation between molecular weight and vapour density of a gas.
(v) It establishes relation between gram molecular weight and grass molecular air volume of a gas.
(vi) Avogadro’s hypothesis modifies Dalton’s atomic theory.
Question 12: A compound is formed by 24 grams of X and 64 grams of oxygen, if X = 12 and O = 16. Find the simplest formula of the compound. ”
Answer:
Therefore, simple ratio between X and O is X : O = 1 : 2
Thus, empirical formula of the compound is XO2.
Question 13: What is the important information given by a balanced chemical equation of decomposition of hydrogen peroxide ?
Answer: The decomposition of hydrogen peroxide is given by the equation:
2H2O2 —> 2H2O + O2
The equation gives the following important information:
(i) Hydrogen peroxide decomposes to form water and oxygen.
(ii) Two molecules of hydrogen peroxide when decomposed, form two molecules of water and one molecule of oxygen.
(iii) 68 gm of hydrogen peroxide when decomposed, yield 36 gm of water and 32 gm of oxygen.
Question 14: What are the limitations of a chemical equation ?
Answer: Following are the limitations of a chemical equation:
(i) A chemical equation does not tell us the physical state of the reactants and the products in the reaction.
(ii) It does not tell us the actual concentration or dilution of the reactants used in the reaction.
(iii) It does not tell whether the reaction starts at its own or some heat is required to start the reaction.
(iv) It does not tell whether the reaction is violent in nature or not.
(v) The time taken by the reaction to complete itself is also not known.
Reasoning Based Questions
Question 1: “When stating the volume of a gas, the pressure and temperatrue should be also given.” Why ?
Answer: It is because, the volume of a gas changes, if the pressure or temperature or both change. Thus, while stating the stating the volume of a gas pressure and volume has to be specified along with volume.
Question 2: “The number of atoms in one mole of hydrogen is twice the number of atoms in 1 mole of helium at the same temperature and pressure.” Why ?
Answer: It is because, hydrogen is a diatomic gas, whereas helium is monoatomic gas. As number of atoms in one molecule of hydrogen are double, as compared to one molecule of helium, therefore one mole of hydrogen has double the atoms,, as compared to helium at the same temperature and pressure.
Question 3: Why is the term relative atomic mass used for atomic mass of an element ?
Answer: Since the actual mass of an atom of element is extremely small, for comparing the masses of atoms of different elements, the mass of an atom of some light element is fixed as standard and the masses of other atoms are expressed relative to the standard mass, so the term relative atomic mass is used.
Question 4: Why relative atomic mass is compared with 1 /12 of carbon ?
Answer: Naturally occurring hydrogen has three isotopes (1H1, 1H2 and 1H3) and its relative atomic mass works out 1.008 rather than 1. Atomic mass of carbon is 12. Thus, 1/12 mass of carbon work out as 1. It is on account of the above reason 1/12 mass of carbon is used for comparing relative atomic masses of other elements.
Question 5: Why atomic weight of many elements are fractional ?
Answer: The atomic weight of all the elements, is never whole number but the atomic weights of many elements are fractional. The atomic weight of an element is fractional because the atomic weight of an element is actually the average relative weight of all the naturally occurring isotopes of the element.
Comments
Post a Comment